Agarose gel
.5 g agarose in 50 mL of 1X TAE (final power of agarose 1% w/v)
Heat on hot plate until moving boil, let awesome for ten minutes
Add ethidium bromide to some final power of .5 µg/mL before flowing gel
Ampicillin 1000X
5. g ampicillin in H2O, 50 mL final volume (100 mg/mL final concentration)
Filter through .22 µm filter
Make 1 mL aliquots, store at -20C
Dilute to 100 µg/mL in LB for microbial cultures
APS (ammonium persulfate)
5 g APS in H2O, 50 mL final volume (10% w/v final concentration)
Make aliquots, store at -20C
DTT (dithiothreitol)
3.86 g of DTT in H2O, 25 mL final volume (1 M final concentration)
Filter through .22 µm filter
Make 1 mL aliquots, store at -20C
IPTG (isopropyl β-D-1-thiogalactopyranoside)
5.96 g of IPTG in H2O, 25 mL final volume (1 M final concentration)
Filter through .22 µm filter
Make 1 mL aliquots, store at -20C
Kanamycin 1000X
1.25 g of kanamycin in H2O, 25 mL final volume (50 mg/mL final concentration)
Filter through .22 µm filter
Make 1 mL aliquots, store at -20C
Dilute to 50 µg/mL in LB for microbial cultures
LB (Luria broth) media
10 g Bacto-tryptone
5 g yeast extract
10 g NaCl
Add 1 L H2O
Autoclave
10X PBS (1 L)
270 mM KCl (20.1 g)
100 mM Na2HPO4 (14.2 g)
18 mM KH2PO4 (2.45 g)
pH 8.
PMSF (phenylmethylsulfanoxide)
.87 g by 50 percent-propanol (isopropanol), 50 mL final volume (100 mM final concentration)
Filter through .22 µm filter
Make 5 mL aliquots, store at -20C
Warm and vortex to create all dissolve, dilute to at least one mM in cell suspension
SDS-PAGE destaining solution
300 mL methanol (30%)
100 mL acetic acidity (10%)
600 mL H2O
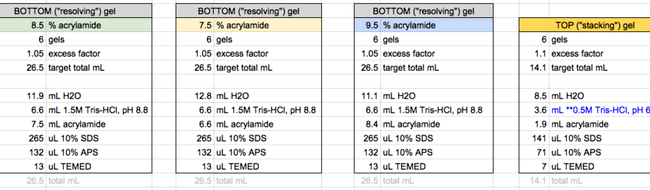
SDS-PAGE gel making buffer
1.5 M Tris-HCl (for separating gel)
118.2 g of Tris-HCl in H2O, pH 8.8
Final volume 500 mL
Filter and degas
SDS-PAGE gel making buffer
1 M Tris-HCl (for stacking gel)
78.8 g of Tris-HCl in H2O, pH 6.8
Final volume 500 mL
Filter and degas
SDS-PAGE 10X gel running buffer
248 mM Trisma (60 g)
1.92 M glycine (288 g)
1% w/v SDS (20 g)
Final volume 2 L
You don't need to pH, filter, or degas
Dilute to 1X for running SDS-PAGE gels
SDS-PAGE marker buffer
4.8 mL of H2O
1.2 mL of just one M Tris-HCl pH 6.8
1 mL of 100% glycerol
2 mL of 10% w/v SDS (sodium dodecyl sulfate)
.5 mL of .1% w/v bromophenol blue
SDS-PAGE marker
25 µL of marker (Bio-Rad catalog number 161-0317)
25 µL of two-mercaptoethanol (BME)
450 µL of SDS-PAGE marker buffer
Heat at 95C for five minutes and store at -20C
4X SDS-PAGE sample loading buffer
1.5 mL of just one M Tris-HCl pH 6.8
3 mL of just one M DTT (dithiothreitol)
.6 g of SDS (sodium dodecyl sulfate)
.03 g of bromophenol blue
2.4 mL of glycerol
Bring final volume to 7.5 mL
If option would be orange/yellow colored, add 1 drop of 5 M NaOH to regulate pH
Make 500 µL aliquots and store at -20C
SDS-PAGE Coomassie staining solution
1.25 g Coomassie R-250
225 mL methanol
225 mL H2O
50 mL glacial acetic acidity
50X TAE buffer for agarose gels
242 g Trisma
20.81 g EDTA
57.1 mL glacial acetic acidity
Need not be pH adjusted
Final volume 1 L