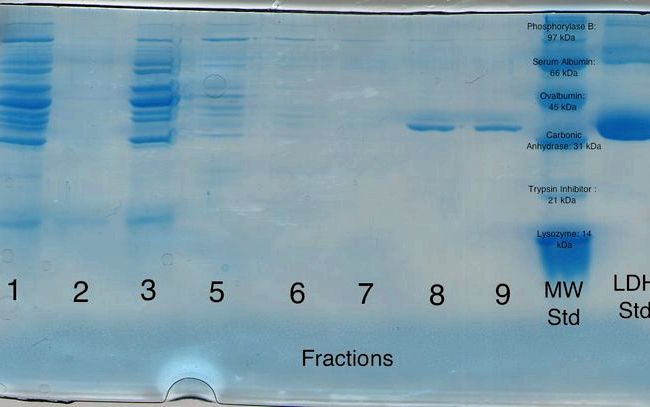
TRICINE GELS Protocol
For Low MW proteins (25000) and peptides
Shagger H. et al. (1987), Rectal Biochem.166: 368-379 and SDS Electrophoresis Techniques - Memebrane Protein Purification and Crystallization
An Operating Guide, 2003, Elsevier Science.
Shagger H (2006) NATURE PROTOCOLS Vol 1 # 1: 16-23 Tricine-SDS- PAGE(pdf)
1) Gel Buffer: 3 M TrisHCl pH8.45 + .3% SDS. Keep RT. (Prepare: 18.15gr Tris + 150mg SDS + H2O as much as 50ml. Adjust pH before you decide to
add some SDS)
2) 40% Acrylamide solution 29:1 (Acrylamide 29 / Methylene bis Acrylamide 1) or 40% Acrylamide solution 19:1 (Acrylamide 19 / Methylene
bis Acrylamide 1) for brief peptides. Keep 4C.
3) Glycerol 70%
4) Cathode buffer 10X (upper buffer): 1M Tris + 1M Tricine + 1% SDS pH 8.25. Keep RT. (Prepare: 24.2gr Tris + 35.84gr Tricine + 2gr SDS
+H2O as much as 200ml. Adjust pH 8.25 before you decide to add SDS).
5) Anode buffer 10X (lower buffer): 2.1M Tris pH8.9 Keep RT. (Prepare: 48.4gr Tris. Adjust pH 8.9 and H2O as much as 200ml).
6) 10% Ammonium Persulfate (APS). Keep under 30 days at 4C.
7) TEMED
Sample Buffer x5
Add TEMED and APS in the finish. Lightly swirl the flask to combine, fostering to not generate bubbles. Pipette the reply to an amount of 4cm of
Fill each sandwich with stacking gel solution and insert a comb into each place being careful to not trap any bubbles bellow one's teeth. The gel should fully polymerized after 1hour. Cover gel having a wrap nylon. Keep gels a maximum of 2 days at 4C. Running conditions: 200 Volts constant.
Just before adding the sample buffer, keep samples at 0C. Add some SDS sample buffer (RT) towards the sample (still on ice), and boil at 100C immediately three to five min. Don't leave the sample in SDS sample buffer without heating endogenous proteases are extremely active in SDS sample buffer and may cause severe degradation. Once heated, sample could spend time at RT for a short while until loading, or at -20C for any lengthy time.
For any gel thickness of .75mm and 15 wells applied 10-25ug protein of the complex mixture, when staining with Coomasie Blue and .5 to 5ug for samples for just one or couple of proteins. If silver stain can be used 10 to 100-fold less protein may be used.
Samples could be concentrated or interferences (salts, etc.) eliminated with TCA, acetone, TCA-DOC, ethanol, etc. (see attached Protocol). Potassium ions particularly should be removed given that they precipitate the SDS.
Some proteins for example nuclear non-histone proteins and membrane proteins, require the existence of 8M urea within the SDS sample buffer to obtain complete solubilization.
Some membrane bound proteins undergo aggregation at temperatures above 40-50 C. Within this situation incubate 30min at 40 C with sample buffer.
A transfer of the migration distances of proteinswith internal disulfide bridgescould be viewed by incubating samples in SDS within the presence or lack of reducing agents (mercaptoethanol, DTT, DTE, etc)
Running conditions: Start running at 60mA 200V 10minutes (to obtain samples inside stacking gel), after which continue at 35mA 200V