By Anne Marie Helmenstine, Ph.D. Chemistry Expert
Anne Helmenstine, Ph.D. is definitely an author and consultant having a broad scientific and medical background. Find out more
Updated May 03, 2016.
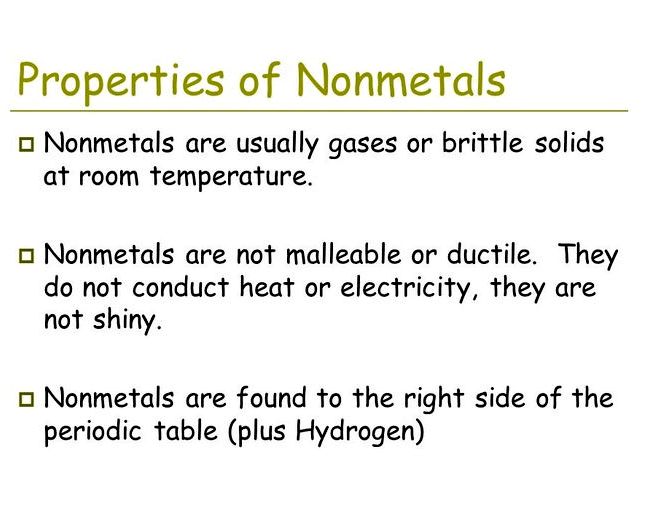
So far as elements are worried, a nonmetal is just a component that doesn't display the qualities of the metal. It's not based on what it's, but by what it's not. It doesn't look metallic, can't be attracted right into a wire or pounded fit or bent, doesn't conduct heat or electricity well, and doesn't have a superior melting or boiling point.
The nonmetals have been in the minority around the periodic table, mostly pressed right hands side from the periodic table.
Continue Studying Below
The exception is hydrogen, which works as a nonmetal at 70 degrees and pressure and it is located on the upper left corner from the periodic table. Here's a glance at which elements are nonmetals, where to find the nonmetals up for grabs, as well as their common qualities.
Location around the Nonmetals around the Periodic Table
The nonmetals are on the upper right side from the periodic table. Nonmetals are separated from metals with a line that cuts diagonally with the region from the periodic table that contains elements with partly filled p orbitals.
The halogens and noble gases are nonmetals, however the nonmetal element group is frequently thought to contain the next elements:
The halogen elements are:
- fluorine
- swimming pool water
- bromine
- iodine
- astatine
- Possibly element 117, although most scientists think this element will become a metalloid.
The noble gas elements are:
Qualities of Nonmetals
Nonmetals have high ion technology powers and electronegativities. They can be poor conductors of warmth and electricity.
Continue Studying Below
Solid nonmetals are usually brittle, with little if any metallic luster. Most nonmetals be capable of gain electrons easily. Nonmetals display an array of chemical qualities and reactivities.
Review of Common Qualities
- High ion technology powers
- High electronegativities
- Poor thermal conductors
- Poor electrical conductors
- Brittle solids - not malleable or ductile
- Little if any metallic luster
- Gain electrons easily
- Dull, not metallic-shiny, although they might be colorful
- Lower melting points and boiling point compared to metals
Evaluating the Metals and Nonmetals
Here's an evaluation from the physical and chemical qualities from the metals and nonmetals. These qualities affect the metals generally (alkali metals, alkaline earth, transition metals, fundamental metals, lanthanides, actinides) and nonmetals generally (nonmetals, halogens, noble gases).